Authors: Maureen Murphy-Ryan, BS | Dr. Paul (Apostolos Psychogios), MD |
Noralane M. Lindor, MD
Abstract
Purpose
To create a practical desk reference for clinicians focused on the differential diagnosis of individuals presenting with features that suggest an inherited disorder of connective tissue.
Methods
We searched the medical literature for distinct clinical entities that shared clinical features with Marfan syndrome and other classical inherited disorders of connective tissue.
Results
Thirty-six distinct heritable disorders of connective tissue were identified that have overlapping features. These disorders were organized into two matrices according to clinical characteristics and according to causative genes.
Conclusions
A broad differential diagnosis is emerging for individuals presenting with features suggestive of altered connective tissue. Recent advances in molecular genetics have aided in the delineation of these disorders.
Main
Many clinicians have a basic knowledge of Marfan syndrome, a relatively common genetic disorder of connective tissue with clinical manifestations involving the musculoskeletal, cardiovascular, respiratory, ophthalmologic, and cutaneous systems.1. However, it is less well known that there is an emerging and complex differential diagnosis to be considered for patients evaluated for a possible heritable disorder of connective tissue. Molecular genetic discoveries have greatly advanced the ability to correctly classify these disorders, and recent reviews have compared clinical features of subsets of the inherited connective tissue disorders.2., 3., 4., 5. The aim of this article was to consolidate new clinical and genetic information in an accessible, comprehensive format to aide in the diagnostic evaluation of individuals who have features that may initially raise the possibility of a connective tissue disorder. The accompanying tables will provide the majority of necessary information to develop a thorough differential diagnosis. Limited text is included to add relevant syndrome-specific information that could not be adequately captured in table format.
Background
Materials and Methods
We developed a graphical format to consolidate the key clinical and genetic features of heritable disorders of connective tissue. Marfan syndrome was used as a platform for comparison. Each of the clinical features of Marfan syndrome, as specified by the revised Ghent Criteria, were subjected to PubMed and OMIM literature searches to identify other Mendelian syndromes or clinical entities that shared that feature. Thirty-six different clinical entities that might share one or more of the presenting features in Marfan syndrome were identified and included in the matrices, involving more than 40 different genes or gene loci. As some of these clinical entities are very new or very rare, only limited data may be available. Boxes are marked with an “x” when that sign was fairly well established in the medical literature as a nonrandom association with that disorder. In Table 1, we present a list of clinical signs that were shared between at least two of the identified syndromes organized by organ system (available as Supplemental Digital Content 1, http://links.lww.com/GIM/A111). Table 2 shows the causative genes for these clinical entities (available as Supplemental Digital Content 2, http://links.lww.com/GIM/A112). This format best illustrates that there is no one-to-one correspondence between genes and disorders.
Table 1Clinical features of disorders of connective tissue (also available at: http://links.lww.com/GIM/A111)
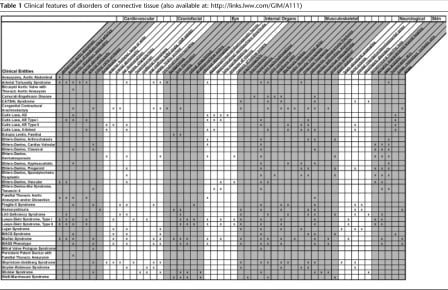
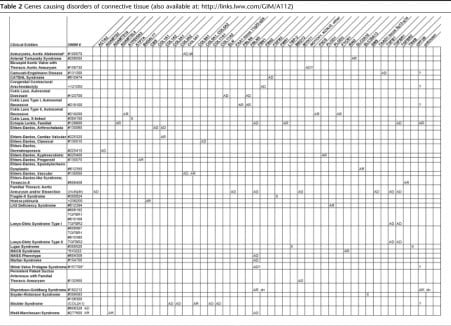
Table 2Genes causing disorders of connective tissue (also available at: http://links.lww.com/GIM/A112)
Results
Aneurysms, aortic abdominal
Arterial tortuosity syndrome
Bicuspid aortic valve with thoracic aortic aneurysm
and research in mice and humans suggests that these conditions have a common etiology through genes such as NOTCH1.13., 14., 15.
Individuals with normal tricuspid aortic valves in families with BAV/TAA can also be at risk for aortic valve calcification or TAA, especially if they carry a known familial mutation in NOTCH1.16.
Clinical management suggestions for BAV/TAA may include pharmacologic therapy with beta-blockers and angiotensin converting enzyme inhibitors or angiotensin receptor blockers, aggressive hypertension control, annual imaging of ascending aortas with diameters >4.0 cm, and elective surgical repair of dilated aortas >5.0 cm in diameter or >4.5 cm with additional risk factors.16.
Camurati-Engelmann disease
CED and Ribbing disease (OMIM no. 601477) are phenotypic variations of the same genetic disorder.
CATSHL syndrome
Generally, strong genotype-phenotype correlations characterize FGFR-related disorders. Mutations in FGFR3 predominantly affect bones that develop by endochondral ossification, whereas mutations in closely related genes, FGFR1 and FGFR2, exert their primary effects on bones developing by intramembranous ossification. FGFR3 mutations may promote (as in the case of CATSHL) or inhibit (as in the achondroplasias) endochondral bone growth.
Congenital contractural arachnodactyly
Significant clinical differences have not been observed between FBN2-mutation-positive and FBN2-negative patients, suggesting locus heterogeneity.20.
Of FBN2-positive patients, frequency of joint dislocation is significantly higher with FBN2 missense mutations compared to splice-site mutations.21.
The proportion of de novo cases is unknown, but many patients diagnosed with CCA have an affected parent.22.
Cutis laxa, autosomal dominant
Cutis laxa, autosomal recessive type I
The genetic basis for the majority of ARCLI cases remains unknown. Recently, Morava et al.28.
published a comprehensive comparison of the autosomal recessive cutis laxa syndromes.
Cutis laxa autosomal recessive Type II
Analysis of apolipoprotein C-III isoelectric focusing, demonstrating a disturbance of O-glycosylation, is diagnostic, and isoelectric focusing of transferrin may also be disturbed. Evaluation of protein glycosylation status is recommended for all children presenting with congenital wrinkled skin/cutis laxa, late fontanel closure, developmental delay, and variable CNS involvement. Wrinkly skin syndrome (OMIM no. 278250) and ARCLII are part of a spectrum of phenotypic variation caused by defects in a single gene.31.
The phenotype may also overlap with gerodermia osteodysplasia (OMIM no. 231070) and de Barsy syndrome (OMIM no. 219150).
Cutis laxa, X-linked
Mental retardation may or may not be present. XLCL is a copper transport disease allelic with Menkes Disease (OMIM no. 309400), often sharing the feature of brittle hair. Serum ceruloplasmin may be low or normal, and lysyl oxidase activity may also be decreased.
Ectopia lentis, familial
Glaucoma can be present secondary to lens subluxation. Although mutations in FBN1 result in autosomal dominant isolated ectopia lentis, autosomal recessive variations have also been described, some of which are due to mutations in LTBP-232.
and ADAMTSL433.
. Three families with autosomal recessive ectopia lentis due to mutations in LTBP-2 exhibited mild-to-moderate osteopenia in carriers and affected individuals as well as a high arched palate, although no other features overlapping with Marfan syndrome were present, whereas all cases with LTBP-2-null mutations in a fourth family had Marfanoid skeletal and joint features without osteopenia.32.
Ectopia lentis may also occur in combination with mild skeletal symptoms, mitral valve prolapse, or nonprogressive aortic root dilatation, especially when due to mutations in FBN1. Some patients diagnosed with familial ectopia lentis may develop aortic aneurysm later in life and could be considered to have late forms of Marfan syndrome. Ectopia lentis has also been identified in a subset of patients with TGFBR2 mutations and minimal skeletal findings.34.
Ehlers-Danlos syndrome, arthrochalasia type (EDS type VIIA, VIIB)
The pathologic basis of this disorder is an inability of α-1 or α-2 chains of type I procollagen to be converted to collagen due to mutations altering the procollagen cleavage site.
Ehlers-Danlos syndrome, cardiac valvular type
Cardiac-valvular EDS is a relatively mild variant on the spectrum of clinical phenotypes resulting from COL1A2 mutations, which also includes osteogenesis imperfecta. Unlike for osteogenesis imperfecta, no skeletal abnormalities are observed in cardiac valvular EDS. The cardiac valvular type of EDS may overlap with mild form of hypermobility type EDS during childhood, with cardiac valvular complications emerging during adulthood.37.
Ehlers-Danlos syndrome, classical type (EDS type I, II)
Type V collagen defects (COL5A1 and COL5A2) are identified in ∼50% of classical EDS cases. For the major and minor diagnostic features of the six commonly recognized types of EDS, see the revised nosology created by the Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK).39.
Ehlers-Danlos syndrome, dermatosparaxis type (EDS type VIIC)
Similar to the arthrochalasia type of EDS, the dermatosparaxis type is caused by deficiencies in the processing of procollagen into collagen. Although the arthrochalasia type of EDS is caused by mutations in COL1A1 or COL1A2, the dermatosporaxis type involves mutations in the gene for the protease ADAMTS-2.41.
Ehlers-Danlos syndrome, kyphoscoliotic type (EDS type VI)
Kyphoscoliotic EDS should be considered if results of neuromuscular tests come back normal, contractures are absent, and Marfanoid habitus is present. Diagnosis of kyphoscoliotic EDS can be biochemically confirmed by a significantly increased ratio of total urinary lysyl pyridinoline to hydroxylysyl pyridinoline.43., 44.
Ehlers-Danlos syndrome, progeroid type
As its name suggests, this type of EDS may be characterized by a distinctive progeroid facies as well as facial skin wrinkling, fine curly hair, scanty eyebrows and eyelashes, and downslanting palpebral fissures. Developmental delay and skeletal abnormalities are also typical. Skin may be loose but still elastic. A spectrum of severity is seen in the skin phenotype and may be as mild as slight facial wrinkling and no remarkable skin laxity.46.
Ehlers-Danlos syndrome, spondylocheirodysplastic type
Ehlers-Danlos syndrome, vascular type (EDS type IV)
Skin hyperelasticity and joint hypermobility are limited in vascular EDS. The majority of familial vascular EDS cases are inherited in an autosomal dominant fashion, although a case of recessively inherited vascular EDS has been identified.50.
There is significant phenotypic overlap between vascular EDS and familial arterial aneurysms. It is important to distinguish between Loeys-Dietz Syndrome and vascular EDS because of the significantly higher rate of intraoperative mortality associated with the latter.51.
Ehlers-Danlos-like syndrome, tenascin X deficiency
Although this is a recessive condition, up to 2/3 of females heterozygous for TNX deficiency may have isolated joint hypermobility. Less invasive alternatives to endoscopy should be considered for patients with TNX deficient EDS because of the theoretical risk of bowel perforation. Baseline pulmonary testing is recommended for all patients with TNX deficient EDS, and smoking patients should be strongly encouraged to stop because of the risk for COPD.53.
Contiguous gene deletions involving both tenascin X and 21-hydroxylase genes can result in congenital adrenal hyperplasia with tenascin X deficiency.54., 55.
Familial thoracic aneurysm and/or dissection
Cerebral vessels may also be affected with fusiform aneurysms or tortuosity. Multiple genes, the majority of which act in an autosomal dominant manner, cause FTAAD, and linkage to other loci indicates that additional genes await discovery. Smooth muscle cell contraction has been found to be important in maintaining the structural integrity of the thoracic/ascending aorta, and mutations in the genes coding for smooth muscle cell α-actin (ACTA2) and β-myosin heavy chain (MYH11) have been identified as causes of FTAAD.57.
Mutations in ACTA2 are the most common cause of FTAAD and may be associated with livedo reticularis, iris flocculi, PDA, and BAV. FTAAD caused by TGBFR mutations often involves skeletal manifestations of a connective tissue disorder (such as joint hypermobility, pes planus, dolichocephaly, or a highly arched palate) that do not meet diagnostic criteria for a particular syndrome. Differences in clinical presentation between individuals with FTAAD due to TGFBR1 and TGFBR2 have been identified, including an increase in cancer incidence with TGFBR1 compared with TGFBR2 and better survival for women with vascular disease due to TGFBR1 mutation with no gender difference seen in TGFBR2.58.
An inclusive overview of the molecular basis of FTAAD was published by Milewicz et al.59.
in 2008.
Fragile-X syndrome
Pes planus is a common musculoskeletal finding in addition to those listed in Table 1 (Supplemental Digital Content 1, http://links.lww.com/GIM/A111). Facial features may be subtle; the characteristic facies is long with a high forehead, large jaw and prominent chin, and ears are characteristically large and low set. Strabismus (but not blue sclera) was present in roughly half of affected individuals in one study.60.
Affected individuals may display features of autism. Behavioral-cognitive manifestations most commonly include a combination of poor eye contact, perseverative speech, echolalia, poor attention span, hyperactivity, and unusual hand mannerisms; a patient who presents with this characteristic facies, joint laxity, macroorchidism, a family history of mental retardation, and any of the psychiatric manifestations listed above should be evaluated for Fragile-X syndrome. Female Fragile X premutation carriers are at risk for premature ovarian failure. Premutations are also associated with a syndrome of tremor and ataxia.62.
Homocystinuria
Clinical clues to the diagnosis include generalized hypopigmentation, pancreatitis, malar flush, and livedo reticularis. Subluxation of the lens is usually downward in homocystinuria, whereas in Marfan syndrome, the lens is generally dislocated upward. Neuropsychiatric disease is common in untreated patients.
LH3 deficiency syndrome
Loeys-Dietz syndrome
In both types of LDS, the vascular manifestations tend to be more severe than in Marfan syndrome and require more aggressive screening and treatment. Pneumothorax is the primary lung manifestation of Loeys-Dietz syndrome. Organs including the spleen, bowel, and uterus are at risk for rupture. Hernias are often recurrent. In addition to dural ectasia, neuroradiologic findings may include Arnold-Chiari type I manifestation, but this may be relatively rare. A minority of LDS patients will have developmental delay.
Lujan syndrome
The major structural defect of the brain is some degree of corpus callosum agenesis. The characteristic facies of Lujan syndrome is long and thin with a tall forehead, short philtrum and sometimes micrognathia. Other distinctive features not mentioned in Table 2 include a hypernasal voice and macrocephaly. For more information on the differential diagnosis for Lujan Syndrome, see Van Buggenhout and Fryns67.
and Staholpu et al.68.
FG syndrome type 1, also known as Opitz-Kaveggia syndrome type 1, is allelic to Lujan syndrome69.
and shares many clinical features, but none that are characteristically marfanoid. Anal anomalies or constipation are restricted to FG Syndrome, and tall stature, long hands and fingers, and hypernasal speech resulting from a high nasal root are more characteristic of LS.70.
Lujan syndrome and FG syndrome are caused by mutations in MED12. Mutations in UPF3B have also been identified in families with Lujan-Fryns phenotype and the FG phenotype.71.
MACS syndrome (macrocephaly, alopecia, cutis laxa, and scoliosis)
The clinical presentation is expected to evolve as more individuals with RIN2 mutations are identified. Macrocephaly and retrognathia are the predominant cranial abnormalities. Alopecia refers to the sparse scalp hair seen in this disorder. Cutis laxa is most notable in the face and mild ichthyosis may also be present. Severe scoliosis appears in the second decade of life (not at birth as in kyphoscoliotic EDS), resulting in short stature. The characteristic facies includes downslanting palpebral fissures, puffy eyelids, sagging cheeks, and everted lower lip. Ortho- and periodontal manifestations include abnormal positioning of the teeth (irregularly placed or unerupted) and gingival hyperplasia. The cutaneous phenotype becomes more pronounced with age in contrast to ARCLII, in which skin manifestations attenuate over time. Skin biopsy of affected individuals revealed fibulin-5 deficiency and a decrease in dermal microfibrils. Facial features resemble GAPO (growth retardation, pseudoanodontia, optic atrophy) syndrome (OMIM no. 230740), a rare autosomal recessive disorder that is phenotypically more severe and includes eye abnormalities, and the dermatosparaxis type of EDS, which also shares gingival hyperplasia but differs in having transparent, easy bruising skin.
Marfan syndrome
Skeletal findings include tall stature relative to other family members (attributed at least in part to disproportionately long limbs), long digits (arachnodactyly), anterior chest deformity including protrusion (pectus carinatum) or sunken appearance (pectus excavatum) of the sternum and anterior ribs which is related to overgrowth of ribs, joint laxity or contractures, scoliosis, and craniofacial manifestations including highly arched palate, crowded teeth, and overbite. Dural ectasia is a major diagnostic clinical criterion involving the lumbosacral spine. Ophthalmologic findings include myopia, lens subluxation (ectopia lentis), and increased axial globe length and corneal flatness. Cardiovascular findings include mitral valve prolapse, mitral regurgitation, aortic regurgitation, and dilatation of the aortic root at the level of sinuses of Valsalva that may result in an aneurysm and/or dissection. Cutaneous features include striae and recurrent hernia formation. Respiratory involvement may manifest as pulmonary blebs, which predispose to pneumothorax. FBN1 mutations are inherited in about two-thirds of cases and rise de novo in the remainder. Hundreds of FBN1 mutations have been described, with the number continuing to grow. Few genotype-phenotype correlations have been established because of the extreme allelic heterogeneity within Marfan syndrome and genetically related disorders.74., 75., 76., 77.
Although there is no known genetic heterogeneity in Marfan Syndrome, TGFBR2 mutations have also been recognized in patients with Marfan-like phenotypes. Clinical outcomes appear similar to those of patients with FBN1 mutations, with prognosis in either group depending more on the clinical disease expression and treatment than the responsible gene. Patients with FBN1 mutations were found to have more extensive skeletal involvement, whereas patients with TGFBR2 mutations had more severe aortic phenotypes overall.34.
MASS phenotype
Consequently, it is difficult to distinguish MASS phenotype from the early stages of Marfan syndrome when assessing individuals, particularly children, in the absence of family history. Intermittent cardiovascular monitoring is therefore indicated.
Mitral valve prolapse syndrome
MVP may be sporadic or familial, isolated, or as part of a syndrome.80.
To date, no specific genes for MVP have been described, although three autosomal and one X-linked loci have been identified. Prognosis in MVP syndrome is better than for MVP in Marfan syndrome, with significantly lower risk for mitral regurgitation.
Persistent patent ductus arteriosis with familial thoracic aneurysm
Risk in patients with PDA with FTA may be assessed through noninvasive measurement of aortic compliance and distensibility using MRI. FTA without PDA is also caused by MYH11 mutations. MYH11 is only rarely involved in sporadic isolated PDA.84.
Shprintzen-Goldberg syndrome
The characteristic facies in SGS can include hypertelorism, down-slanting palpebral fissures, a highly arched palate, micrognathia, proptosis (protruding eyes), and low set, posteriorly rotated ears. The presence of this characteristic facies, craniosynostosis, and mental retardation are the major features used to distinguish SGS clinically. Other features include Chiari malformation, rib anomalies, and equinovarus deformity (clubfeet). SGS is distinct from Goldberg-Shprintzen syndrome (OMIM no. 609640) and Shprintzen syndrome (OMIM no. 192430).
Snyder-Robinson syndrome
Stickler syndrome
Two vitreoretinal phenotypes are clinically recognized.93.
Mutations in COL2A1 are associated with a type 1 “membranous” vitreous phenotype. Because exon 2 of COL2A1 is preferentially expressed in the eye, mutations in this exon result in an isolated type 1 vitreous defect.94.
The less common type II “beaded” vitreous phenotype is associated with mutations in COL11A1. Mutations in COL11A2 produce a Stickler phenotype lacking vitreous manifestations, because COL11A2 is not expressed in this tissue.95.
Weill-Marchesani syndrome
There was approximately a 20% difference in incidence between AR and AD WMS for microspherophakia and cardiac anomalies (more prevalent in AR WMS) as well as joint limitations and ectopia lentis (more prevalent in AD WMS),97.
although these characteristics are commonly seen in both forms of WMS. Further complicating differentiation between AR and AD WMS based on family history, some heterozygotes for AR WMS display mild clinical manifestations of the disease, including short stature, brachydactyly, and abnormal gonioscopic findings. In addition to the eye manifestations listed in Table 2 (Supplemental Digital Content 2, http://links.lww.com/GIM/A112), shallow orbits and glaucoma can be features of WMS. Congenital cardiac abnormalities reported for patients with WMS include MVP, prolonged QT interval, and pulmonary and aortic valve stenoses. Patients with WMS and a history of palpitation, lightheadedness, dizziness or syncope should be evaluated for prolonged QTc, as it may be associated with serious arrhythmia.98.
The differential diagnosis of WMS includes Hunter-MacDonald Syndrome.99.
Discussion
-
Evaluation by an ophthalmologist with knowledge of disorders of connective tissue to assess abnormalities of the lens, retina, vitreous, and refraction.
-
Radiographic imaging, including transthoracic or esophageal echocardiography, with careful, serial measurements of the aortic root at the level of the sinuses of Valsalva to compare with normal references for age and/or body mass index and evaluation of the aortic arch.
-
CT or MR angiogram of the cerebral, neck, thoracic, abdominal, and pelvic arteries. An abnormality such as dissection, dilation, tortuosity, or aneurysm in any one artery should prompt a systematic assessment of all major arteries.
-
A skeletal survey that may help evaluate underlying skeletal dysplasia, acetabular abnormalities, hyperostosis, or osteopenia.
-
A bone densitometry study may be useful for identifying osteopenia.
-
Special spine films, to help accurately diagnose the degree of any scoliosis.
-
Brain imaging that may be indicated in individuals with neurologic signs or symptoms but not usually in individuals lacking such neurological features.
-
Audiologic assessment, recommended when disorders with hearing loss are included in the differential diagnosis list.
-
Clinical molecular testing when available for genes included in Table 2 (Supplemental Digital Content 2, http://links.lww.com/GIM/A112) associated with the disorders under consideration in the differential diagnosis. Current information on test availability and links to the laboratories offering testing can be found at Genetests.org.
An accurate diagnosis can guide the physician and patient in monitoring for the progression of known symptoms or emergence of new symptoms, identifying high-risk situations, and identifying other at-risk family members. Standard management recommendations have been published for the more common inherited connective tissue disorders such as Marfan syndrome, and the importance of diagnostic accuracy will increase as further disorder-specific evidence-based treatment regimes are developed. Diagnostic accuracy may improve the quality of life for patients, even those with disorders for which no major treatment has yet been developed. The matrices constructed from this project may be of clinical use to geneticists as well as other clinicians who encounter patients with apparent alterations affecting connective tissue.
Additional information
References
-
The Marfan syndrome: diagnosis and management.N Engl J Med. 1979; 300: 772-777
-
Challenges in the diagnosis of Marfan syndrome.Med J Aust. 2006; 184: 627-631
-
Report of the National Heart, Lung, and Blood Institute and National Marfan Foundation Working Group on research in Marfan syndrome and related disorders.Circulation. 2008; 118: 785-791
-
Ehlers-Danlos syndromes and Marfan syndrome.Best Pract Res Clin Rheumatol. 2008; 22: 165-189
-
Recent progress in genetics of Marfan syndrome and Marfan-associated disorders.J Hum Genet. 2007; 52: 1-12
-
Fibulins: physiological and disease perspectives.EMBO Rep. 2003; 4: 1127-1131
-
The ADAMTS metalloproteinases.Biochem J. 2005; 386: 15-27
-
Fibrillin-1 misfolding and disease.Antioxid Redox Signal. 2006; 8: 338-346
-
Familial abdominal aortic aneurysms: collection of 233 multiplex families.J Vasc Surg. 2003; 37: 340-345
-
Identification of a p.Ser81Arg encoding mutation in SLC2A10 gene of arterial tortuosity syndrome patients from 10 Qatari families.Clin Genet. 2008; 74: 189-193
-
A novel missense and recurrent mutation in SLC2A10 gene of patients affected with arterial tortuosity syndrome.Atherosclerosis. 2009; 203: 466-471
-
Natural history of ascending aortic aneurysm in the setting of an unreplaced bicuspid aortic valve.Ann Thorac Surg. 2007; 83: 1338-1344
-
Mutations in NOTCH1 cause aortic valve disease.Nature. 2005; 437: 270-274
-
Molecular genetics of aortic valve disease.Curr Opin Cardiol. 2006; 21: 180-184
-
Novel NOTCH1 mutations in patients with bicuspid aortic valve disease and thoracic aortic aneurysms.J Thorac Cardiovasc Surg. 2007; 134: 290-296
-
Ascending aortic dilatation associated with bicuspid aortic valve: pathophysiology, molecular biology, and clinical implications.Circulation. 2009; 119: 880-890
-
Camurati-Engelmann disease: review of the clinical, radiological, and molecular data of 24 families and implications for diagnosis and treatment.J Med Genet. 2006; 43: 1-11
-
A novel mutation in FGFR3 causes camptodactyly, tall stature, and hearing loss (CATSHL) syndrome.Am J Hum Genet. 2006; 79: 935-941
-
Familial occurrence of typical and severe lethal congenital contractural arachnodactyly caused by missplicing of exon 34 of fibrillin-2.Am J Hum Genet. 1996; 59: 1027-1034
-
Comprehensive clinical and molecular assessment of 32 probands with congenital contractural arachnodactyly: report of 14 novel mutations and review of the literature.Hum Mutat. 2009; 30: 334-341
-
The FBN2 gene: new mutations, locus-specific database (Universal Mutation Database FBN2), and genotype-phenotype correlations.Hum Mutat. 2009; 30: 181-190
-
FBN2, FBN1, TGFBR1, and TGFBR2 analyses in congenital contractural arachnodactyly.Am J Med Genet A. 2007; 143: 694-698
-
Highly variable cutis laxa resulting from a dominant splicing mutation of the elastin gene.Am J Med Genet A. 2008; 146A: 977-983
-
A novel elastin gene mutation resulting in an autosomal dominant form of cutis laxa.Arch Dermatol. 2004; 140: 1135-1139
-
Aortic aneurysmal disease and cutis laxa caused by defects in the elastin gene.J Med Genet. 2006; 43: 255-258
-
Fibulin-4: a novel gene for an autosomal recessive cutis laxa syndrome.Am J Hum Genet. 2006; 78: 1075-1080
-
Autosomal recessive cutis laxa syndrome revisited.Eur J Hum Genet. 2009; 17: 1099-1100
-
Defining the phenotype in an autosomal recessive cutis laxa syndrome with a combined congenital defect of glycosylation.Eur J Hum Genet. 2008; 16: 28-35
-
Mutation in pyrroline-5-carboxylate reductase 1 gene in families with cutis laxa type 2.Am J Hum Genet. 2009; 85: 120-129
-
Mutations in PYCR1 cause cutis laxa with progeroid features.Nat Genet. 2009; 41: 1016-1021
-
Impaired glycosylation and cutis laxa caused by mutations in the vesicular H+-ATPase subunit ATP6V0A2.Nat Genet. 2008; 40: 32-34
-
Null mutations in LTBP2 cause primary congenital glaucoma.Am J Hum Genet. 2009; 84: 664-671
-
A homozygous mutation in ADAMTSL4 causes autosomal-recessive isolated ectopia lentis.Am J Hum Genet. 2009; 84: 274-278
-
Comparison of clinical presentations and outcomes between patients with TGFBR2 and FBN1 mutations in Marfan syndrome and related disorders.Circulation. 2009; 120: 2541-2549
-
Ehlers-Danlos syndrome type VIIA and VIIB result from splice-junction mutations or genomic deletions that involve exon 6 in the COL1A1 and COL1A2 genes of type I collagen.Am J Med Genet. 1997; 72: 94-105
-
Rare autosomal recessive cardiac valvular form of Ehlers-Danlos syndrome results from mutations in the COL1A2 gene that activate the nonsense-mediated RNA decay pathway.Am J Hum Genet. 2004; 74: 917-930
-
Total absence of the alpha2(1) chain of collagen type I causes a rare form of Ehlers-Danlos syndrome with hypermobility and propensity to cardiac valvular problems.J Med Genet. 2006; 43: E36
-
Molecular genetics in classics Ehlers-Danlos Syndrome.Am J Med Genet C Semin Med Genet. 2005; 139C: 17-23
-
Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK).Am J Med Genet. 1998; 77: 31-37
-
The natural history, including orofacial features of three patients with Ehlers-Danlos syndrome, dermatosparaxis type (EDS type VIIC).Am J Med Genet A. 2004; 131: 18-28
-
Novel types of mutation responsible for the dermatosparactic type of Ehlers-Danlos syndrome (Type VIIC) and common polymorphisms in the ADAMTS2 gene.J Invest Dermatol. 2004; 123: 656-663
-
Differential diagnosis of muscular hypotonia in infants: the kyphoscoliotic type of Ehlers-Danlos syndrome (EDS VI).Neuromuscul Disord. 2008; 18: 906-907
-
Differential diagnosis of muscular hypotonia in infants: the kyphoscoliotic type of Ehlers-Danlos syndrome (EDS VI).Neuromuscul Disord. 2008; 18: 210-214
-
A case of Ehlers Danlos syndrome type VI.Genet Couns. 2006; 17: 291-294
-
The congenital disorders of glycosylation are clinical chameleons.Eur J Hum Genet. 2008; 16: 2-4
-
A novel missense mutation in the galactosyltransferase-I (B4GALT7) gene in a family exhibiting facioskeletal anomalies and Ehlers-Danlos syndrome resembling the progeroid type.Am J Med Genet A. 2004; 128A: 39-45
-
Spondylocheiro dysplastic form of the Ehlers-Danlos Syndrome—an autosomal-recessive entity caused by mutations in the zinc transporter gene SLC39A13.Am J Hum Genet. 2008; 82: 1290-1305
-
Clinical and genetic features of vascular Ehlers-Danlos syndrome.Ann Vasc Surg. 2002; 16: 391-397
-
Stickler syndrome type 2 and linkage to the COL11A1 gene.Ann N Y Acad Sci. 1996; 785: 331-332
-
Homozygosity for a null allele of COL3A1 results in recessive Ehlers-Danlos syndrome.Eur J Hum Genet. 2009; 17: 1411-1416
-
Aneurysm syndromes caused by mutations in the TGF-beta receptor.N Engl J Med. 2006; 355: 788-798
-
Neuromuscular involvement in various types of Ehlers-Danlos syndrome.Ann Neurol. 2009; 65: 687-697
-
Tenascin-X, collagen, elastin, and the Ehlers-Danlos syndrome.Am J Med Genet C Semin Med Genet. 2005; 139C: 24-30
-
A recessive form of the Ehlers-Danlos syndrome caused by tenascin-X deficiency.N Engl J Med. 2001; 345: 1167-1175
-
Tenascin-X deficiency in autosomal recessive Ehlers-Danlos syndrome.Am J Med Genet A. 2005; 135: 75-80
-
Identification of a chromosome 11q23.2-q24 locus for familial aortic aneurysm disease, a genetically heterogeneous disorder.Circulation. 2001; 103: 2469-2475
-
Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections.Nat Genet. 2007; 39: 1488-1493
-
Analysis of multigenerational families with thoracic aortic aneurysms and dissections due to TGFBR1 or TGFBR2 mutations.J Med Genet. 2009; 46: 607-613
-
Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction.Annu Rev Genomics Hum Genet. 2008; 9: 283-302
-
Orthopaedic aspects of fragile-X syndrome.J Bone Joint Surg Am. 1990; 72: 889-896
-
Aspects of skeletal development in fragile X syndrome fetuses.Am J Med Genet. 2000; 95: 123-129
-
Fragile X Syndrome.Eur J Hum Genet. 2008; 16: 666-672
-
Classical homocystinuria: vascular risk and its prevention.J Inherit Metab Dis. 2003; 26: 259-265
-
A connective tissue disorder caused by mutations of the lysyl hydroxylase 3 gene.Am J Hum Genet. 2008; 83: 495-503
-
Phenotypic heterogeneity of Marfan-like connective tissue disorders associated with mutations in the transforming growth factor-receptor genes.Circ J. 2007; 71: 1305-1309
-
Psychopathology in the Lujan-Fryns syndrome: report of two patients and review.Am J Med Genet A. 2006; 140: 2807-2811
-
Lujan-Fryns syndrome (mental retardation, X-linked, marfanoid habitus).Orphanet J Rare Dis. 2006; 1: 26
-
Terminal deletion of chromosome 5p in a patient with phenotypical features of Lujan-Fryns syndrome.Am J Med Genet A. 2003; 119A: 363-366
-
The original Lujan syndrome family has a novel missense mutation (p.N1007S) in the MED12 gene.J Med Genet. 2007; 44: 472-477
-
The mediator of RNA polymerase II.Chromosoma. 2005; 113: 399-408
-
Mutations in UPF3B, a member of the nonsense-mediated mRNA decay complex, cause syndromic and nonsyndromic mental retardation.Nat Genet. 2007; 39: 1127-1133
-
RIN2 deficiency results in macrocephaly, alopecia, cutis laxa, and scoliosis: MACS syndrome.Am J Hum Genet. 2009; 85: 254-263
-
Revised diagnostic criteria for the Marfan syndrome.Am J Med Genet. 1996; 62: 417-426
-
The importance of mutation detection in Marfan syndrome and Marfan-related disorders: report of 193 FBN1 mutations.Hum Mutat. 2007; 28: 928
-
Delineation of the Marfan phenotype associated with mutations in exons 23–32 of the FBN1 gene.Am J Med Genet. 1996; 62: 233-242
-
Fibrillin-1 (FBN1) gene frameshift mutations in Marfan patients: genotype-phenotype correlation.Clin Genet. 2001; 59: 444-450
-
Utility of molecular analyses in the exploration of extreme intrafamilial variability in the Marfan syndrome.Clin Genet. 2007; 72: 188-198
-
Association of mitral valve prolapse and systemic abnormalities of connective tissue: a phenotypic continuum.JAMA. 1989; 262: 523-528
-
Prevalence and clinical outcome of mitral-valve prolapse.N Engl J Med. 1999; 341: 1-7
-
The genetics of mitral valve prolapse.Clin Genet. 2007; 72: 288-295
-
Familial thoracic aortic aneurysm/dissection with patent ductus arteriosus: genetic arguments for a particular pathophysiological entity.Eur J Hum Genet. 2004; 12: 173-180
-
Mapping of familial thoracic aortic aneurysm/ dissection with patent ductus arteriosis to 16p12.2–p13.13.Circulation. 2005; 112: 200-206
-
Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus.Nat Genet. 2006; 38: 343-349
-
Investigation of the MYH11 gene in sporadic patients with an isolated persistently patent arterial duct.Cardiol Young. 2007; 17: 666-672
-
Shprintzen-Goldberg syndrome associated with a novel missense mutation in TGFBR2.Exp Dermatol. 2008; 17: 362-365
-
Mutations in fibrillin-1 and the Marfanoid-craniosynostosis (Shprintzen-Goldberg) syndrome.Nat Genet. 1996; 12: 209-211
-
Molecular pathology of Shprintzen-Goldberg syndrome.Am J Med Genet A. 2006; 140: 104-108
-
Shprintzen-Goldberg syndrome: fourteen new patients and a clinical analysis.Am J Med Genet A. 2005; 135: 251-262
-
A missense mutation, p.V132G, in the X-linked spermine synthase gene (SMS) causes Snyder-Robinson syndrome.Am J Med Genet A. 2009; 149A: 328-335
-
X-linked spermine synthase gene (SMS) defect: the first polyamine deficiency syndrome.Eur J Hum Genet. 2003; 11: 937-944
-
New SMS missense mutation leads to a striking reduction in spermine synthase protein function and a severe form of Snyder-Robinson X-linked recessive mental retardation syndrome.J Med Genet. 2008; 45: 539-543
-
The Stickler syndrome: genotype/phenotype correlation in 10 families with Stickler syndrome resulting from seven mutations in the type II collagen gene locus COL2A1.Genet Med. 2003; 5: 21-27
-
Stickler syndrome: further mutations in COL11A1 and evidence for additional locus heterogeneity.Eur J Hum Genet. 1999; 7: 807-814
-
Clinical variability of Stickler syndrome: role of exon 2 of the collagen COL2A1 gene.Surv Opthalmol. 2003; 48: 191-203
-
Stickler syndrome without eye involvement is caused by mutations in COL11A2, the gene encoding the alpha2(XI) chain of type XI collagen.J Pediatr. 1998; 132: 368-371
-
ADAMTS10 mutations in autosomal recessive Weill-Marchesani syndrome.Am J Hum Genet. 2004; 75: 801-806
-
Clinical homogeneity and genetic heterogeneity in Weill-Marchesani syndrome.Am J Med Genet A. 2003; 123A: 204-207
-
Cardiac findings in Weill-Marchesani syndrome.Am J Med Genet A. 2007; 143A: 2062-2064
-
The Hunter-MacDonald syndrome with expanded phenotype including risk of meningioma: an update and review.Am J Med Genet A. 2008; 146A: 83-92
-
Marfan's syndrome and related disorders—more tightly connected than we thought.N Engl J Med. 2006; 355: 841-844
-
Recent advances in understanding Marfan syndrome: should we now treat surgical patients with losartan?.J Thorac Cardiovasc Surg. 2008; 135: 389-394